Testing, the Wuhan Flu, and Opening Up America
Earlier virus testing probably wouldn’t have changed anything; public private partnership are phenomenal, and there’s still work to be done.
When the After Action Review is written on the current pandemic, expect the thickest chapter to revolve around testing. Trying to wade through the Trump hatred that virulently infects virtually every MSM article about this pandemic, then teasing some objective reality about any aspect of it — including testing — can be a difficult task. Here are the issues surrounding testing, the pandemic, and the plan to reopen America as I see them.
1. The CDC failed in its initial response, but that probably did not have the dire consequences that the progressive left is claiming.
Under the emergency protocol, when faced with a new contagion, CDC is supposed to design and produce a test kit. Health care providers then apply the test and send it to the CDC for processing. Days later, the CDC announces test results.
The Wuhan virus is new, so the CDC did not have existing tests in January 2020. The CDC immediately blew it when developing tests:
The Food and Drug Administration this week said that scientists with the CDC had failed to adhere to containment standards while developing the test, exposing the kits to contamination and setting the rollout of the test back as much as a month. The organization “made its test in one of its laboratories, rather than in its manufacturing facilities,” against its own protocol, the FDA said.
The revelation comes at a time when politicians and administration critics have accused the federal government of falling seriously behind in the number of tests it should be conducting. The U.S. has performed the highest total number of coronavirus tests in the world, though by population numbers it has lagged behind countries such as Belgium, Spain and Germany.
This failure was “a glaring scientific breakdown at the CDC’s central laboratory.”
The troubled segment of the test was not critical to detecting the novel coronavirus, experts said. But after the difficulty emerged, CDC officials took more than a month to remove the unnecessary step from the kits, exacerbating nationwide delays in testing, . . .
Shortcomings with the tests were first noticed in late January, after the CDC sent an initial batch to 26 public health labs across the country. According to those with knowledge of what unfolded, false-positive reactions emerged at 24 of the 26 labs that first tried out the kits in advance of analyzing samples gathered from patients.
“Only two of them got it right,’’ said a senior federal scientist who reviewed the development of the kits and internal test documentation, and who concluded that the false positives were caused by contamination that occurred at the CDC.
The Washington Post gives the progressive line on this treatment:
The failure with testing kept the public health labs from performing disease surveillance intended to predict and minimize harm before the virus became widely established in the United States. The impact has been magnified by the nation’s inability to rapidly expand the availability of testing.
Or as a recent March 10 New York Times primal scream on the topic read, ‘It’s Just Everywhere Already’: How Delays in Testing Set Back the U.S. Coronavirus Response. They add in The Lost Month that the causes were several, including a “lack of leadership.” All Times readers know what that means.
Given what we now know, though, I don’t believe that the progressive media is correct that the U.S. has been devastated because of the CDC’s delay in getting working tests out.
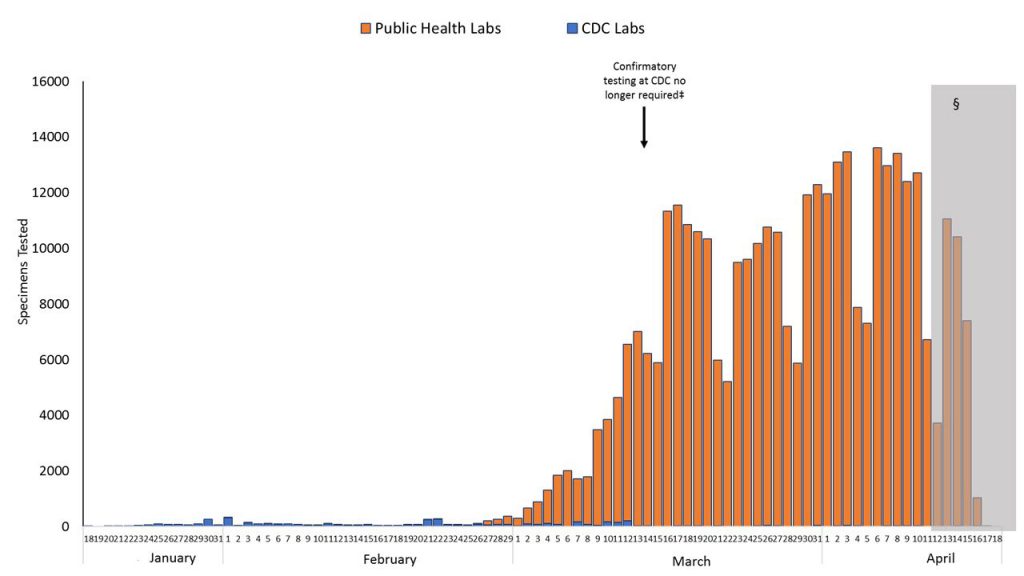
The initial CDC tests, even if all were done perfectly, would still have failed to give Trump, or anyone, a working picture about the virus. The tests would have been far too few in number and taken far too long to process because the CDC ultimately had to confirm each test at their own lab. I do not have the hard numbers, but the CDC graph below gives a visual.
It seems that through February 26, the CDC was only processing tests in the double digits per day. We now know that, in December 2019 and January 2020, the virus was likely running wild through the population in California, Massachusetts and probably elsewhere. We also know that a significant portion of the people infected show few, if any symptoms, so they wouldn’t have been tested under the CDC guidelines in force through February 28 (specific symptoms plus China travel) even if the tests had been perfect. And even if a person was tested, the tests would only have shown if they had an infection on that day, not whether they had previously been infected. Indeed, it wasn’t until April 2 that an antigen test for the presence of antibodies because of prior infection was created and approved in the U.S.
The CDC protocols for responding were insufficient on their face for a virus that moved with the speed and infectiousness of the Wuhan virus. CDC was grossly unprepared. That said, even if the tests were perfect and China had not hidden information about the virus, it does not seem to me that this would have changed our current situation.
2. From the start, regulations and bureaucratic inertia were the real obstacles to a timely response to the virus
Little is more regulated in the U.S. than healthcare, and that includes medical testing. The limiting regulations exist to ensure that medical tests are only approved after regulators validate them and when they are certain to protect patient privacy. Both goals are what we want from our government, but in the unique scenario that the Wuhan virus posed, they became a huge roadblock. Bureaucrats dotted i’s and crossed t’s like they were Volgons at a time when speed, flexibility and decisiveness were desperately needed.
There are two excellent articles on this. One is a March 10 article in the New York Times, ‘It’s Just Everywhere Already’: How Delays in Testing Set Back the U.S. Coronavirus Response. The article is informative with very little Trump Derangement Syndrome (probably because the by-line does not contain the names Michael Shearer or Maggie Haberman). The second is a March 16 article from GQ’s resident Trump-hater, Julia Ioffe, The Infuriating Story of How the Government Stalled Coronavirus Testing. Both tell the first half of the story, the red tape and the bureaucratic inertia, in excruciating detail. For example, from the GQ article:
Greninger decided to keep going in his effort to create a test that the University of Washington could use in-house. On February 18, he submitted a request to the FDA for a preliminary Emergency Use Authorization (EUA), or permission to develop and use his own coronavirus test. Even though he kept quietly working on the test while waiting for permission to come, he understood why the FDA regulated such things: you don’t want bad tests circulating in the healthcare system, giving people false positives of negatives, especially in the midst of a pandemic. Bad tests could be just as dangerous as no tests at all.
What followed, however, went far beyond quality control—and into the realm of the absurd.
After emailing his application to the FDA, Greninger discovered that it was incomplete. It turned out that in addition to electronically filing it, he also had to print it out and mail a physical copy along with a copy burned onto a CD or saved to a thumb drive. That package had to be shipped off to FDA headquarters in Maryland. It was a strange and onerous requirement in 2020, but Greninger complied. He had no choice. On February 20, he overnighted the hard copies of his application to the FDA.
Four days later, the FDA responded with its guidance. It needed some more information and wanted Greninger to conduct a few more tests. According to Greninger, the FDA wanted him to run his SARS-CoV-2 test against some older viruses in his lab to make sure his test didn’t cross-react with them. The officials at the FDA instructed him to test his test against the MERS and SARS viruses, which are also coronaviruses. It wasn’t a terrible idea, Greninger thought. Why not develop a test that catches all these deadly coronaviruses all at once? It did seem strange, however, that the FDA was asking for this in an emergency use application: by this point, COVID-19 cases had appeared in six states. (The FDA did not respond to a list of questions about the process.)
Still, Greninger complied. He called the CDC to inquire about getting some genetic material from a sample of SARS. The CDC, Greninger says, politely turned him down: the genetic material of the extremely contagious and deadly SARS virus was highly restricted.
“That’s when I thought, ‘Huh, maybe the FDA and the CDC haven’t talked about this at all,’” Greninger told me. “I realized, Oh, wow, this is going to take a while, it’s going to take several weeks.” . . .
If you have not, I recommend that you read both articles in full. And as well, for a pithy take on the problem with no TDS, see Tyler Cowen’s February 23 post on the issue at Marginal Revolution. That said, the part that no article that I have seen answers is who was responsible for cutting through the red tape and lighting a fire under the bureaucracy. I would imagine that part of the story is favorable to Trump, and that is why we have not heard of it yet.
Lastly, while I claim no special expertise at all in the legal minutia of health care and testing regulation, it is abundantly clear that our public health officials’ failure to react in a timely manner to the Wuhan virus was largely systemic. Progressives want to paint the failures as a failure of execution by Trump and his administration, but nothing I have read suggests that the Trump administration was involved in the bureaucratic roadblocks to developing tests at any point.
3. The ramp-up in testing by the private sector and pushed by the Trump administration has been phenomenal.
At some point, the Trump administration did the two things that had to be done: Involve the private sector and release the private sector from the stringent bureaucratic restrictions tying them down. As quoted at Instapundit:
“The most striking aspect of the administration’s response has been its waiving or liberalizing of hundreds of regulatory requirements that would otherwise impede efforts to cope with the epidemic and ensuing shutdowns. The Food and Drug Administration has relaxed its extreme restrictions on the development and deployment of medical tests, equipment, drugs and vaccines. The Medicare and Hipaa waivers, along with the suspension by many states of their restrictions on out-of-state medical professionals, are allowing doctors and nurses to go where they are needed and to practice telemedicine. The Education Department is easing its micromanagement of school districts to facilitate online teaching and other initiatives. Teachers I know are enthusiastic about the cancellation of this year’s federal testing requirements—now they can actually teach their students instead of merely preparing them for tests.”
On February 26, President Trump put Vice President Pence in charge of the Coronavirus Task Force. On February 29, the Food and Drug Administration (FDA) allowed certified labs to develop and begin testing coronavirus testing kits while reviewing pending applications. On March 3, the CDC lifted federal restrictions on coronavirus testing to allow any American to be tested for coronavirus, “subject to doctor’s orders.” On March 13, the FDA granted Roche AG an emergency approval for automated coronavirus testing kits and issued an emergency approval to Thermo Fisher for a coronavirus test within 24 hours of receiving the request.
To again show the graph of testing once the CDC restrictions were removed and the private sector enlisted . . .
. . . the impact is stunning. The number of tests has gone from just a few tests per day to over 150,000 per day. Wired has an excellent article on private sector developments that have occurred in just the past month, as well as a discussion of some of the challenges we still face:
. . . A new trend promises to open up these bottlenecks. A wide range of companies are developing tests that can be deployed to hospitals, doctor’s offices, airports and—maybe—even people’s homes. These machines, generally the size of a countertop kitchen appliance, can be used to test patients right at the point of care, yielding results within an hour. The FDA, in full catch-up mode, has recently handed out emergency approvals for four of these decentralized testing platforms, which are starting to expand and diversify testing capabilities. These emergency approvals let companies provide testing based on preliminary data on the devices, before the conclusion of a lengthy FDA review, for as long as the health emergency continues.
On March 20, molecular diagnostics company Cepheid received the first FDA approval for a fast, point-of-care Covid-19 test. The test comes as a $35 flip-top plastic cartridge, about the size of a roll of half dollars, that plugs into the company’s existing GeneXpert devices. . . .
These tests don’t merely increase the numbers of tests that can be run and the speed of the results, but they test for different things relative to the disease, as explained by Drs. Fauci and Birx. I recommend skipping to the 2nd video below when Dr. Birx appears to give her full remarks:
4. Testing is imperfect and the amount of red tape and regulation now cut is showing its own problems.
Oft times when the pendulum swings, it does too far in the other direction. Such may well be the case now where the FDA may have exercised poor judgment in its rush to make testing available. Here’s a rule of thumb for partnering with the private sector . . . Communist China DOES NOT HAVE a private sector. This from the NYT:
In recent weeks, the United States has seen the first rollout of blood tests for coronavirus antibodies, widely heralded as crucial tools to assess the reach of the pandemic in the United States, restart the economy and reintegrate society.
But for all their promise, the tests — intended to signal whether people may have built immunity to the virus — are already raising alarms.
Officials fear the effort may prove as problematic as the earlier launch of diagnostic tests that failed to monitor which Americans, and how many, had been infected or developed the disease the virus causes. Criticized for a tragically slow and rigid oversight of those tests months ago, the federal government is now faulted by public health officials and scientists for greenlighting the antibody tests too quickly and without adequate scrutiny.
The Food and Drug Administration has allowed about 90 companies, many based in China, to sell tests that have not gotten government vetting, saying the pandemic warrants an urgent response. But the agency has since warned that some of those businesses are making false claims about their products; health officials, like their counterparts overseas, have found others deeply flawed.
Tests of “frankly dubious quality” have flooded the American market, said Scott Becker, executive director of the Association of Public Health Laboratories. . . .
The feds need to get a handle on this or it could bloom into a serious problem.
5. Are there sufficient tests for states to begin reopening per the Open America plan?
The Coronavirus Task Force has promulgated the Opening Up America phased plan. The plan is built not merely on testing and trends, but public health monitoring of flu-like illnesses that would almost certainly be due to the Wuhan virus now that flu season is over. Thus there is much more involved in this plan than just individual testing to tell if lifting stay at home orders starts a localized resurgence of the Wuhan virus, in which case the federal and state government would move quickly to that locale to address the issue. As Dr. Fauci explained:
. . . although testing was important, it was just one part of the overall strategy to fight the coronavirus.
“The emphasis we’ve been hearing is essentially testing is everything, and it isn’t,” Fauci said.
Federal public health officials are convinced that there are enough tests for all of the states to begin Phase I of the Opening Up America plan. This from ABC:
Vice President Mike Pence and the administration’s top health officials said the United States has conducted roughly 3.7 million diagnostic tests — roughly 120,000 a day — and argued that the U.S. has the infrastructure to improve testing capacity. . . .
With many states concerned about lacking the testing required to monitor their populations, the administration has outlined efforts to help states expand their efforts.
“There is existing capacity that we have, that for one reason or other maybe has not been fully communicated,” Dr. Anthony Fauci, the nation’s top infectious disease expert, said Friday.
Added Pence: “We actually believe that we could double the amount of testing that’s taking place every day if we simply brought online all of the testing capabilities and all the labs.” . . .
Obviously a part of the problem, particularly for any risk-averse governor, let alone the neo-Marxist proggies among them, is that they are the ones that are going to have to decide when to open their states. The goal for progressive governors (besides using the federal tax dollar to cover their long-term mismanagement) is to keep the blame shifted. And, of course, the MSM is there to help. This from NBC:
Governors across the country on Sunday pushed back on the Trump administration’s claims that states are conducting a “sufficient” level of coronavirus testing.
Speaking with CNN’s “State of the Union,” Virginia Gov. Ralph Northam, a Democrat, said it was “delusional” to suggest the states have enough tests to soon begin reopening their economies.
“That’s just delusional to be making statements like that,” Northam said. “We have been fighting every day for PPE. And we have got some supplies now coming in. We have been fighting for testing. It’s not a — it’s not a straightforward test. We don’t even have enough swabs, believe it or not. And we’re ramping that up. But for the national level say that we have what we need, and really to have no guidance to the state levels, is just irresponsible, because we’re not there yet.” . . .
Something tells me that Coonman is prepared to classify every death in Virginia as a Wuhan flu death, regardless of whether the state tests every single person, and then to blame Trump for all those deaths. It’s ludicrous. Pandemics end when the R0 — i.e., the average number of people being infected by each person sick with the disease — falls below 1.0. The benchmarks of the Opening Up America Plan are designed around achieving and maintaining that R0, not achieving 100% testing of all citizens, something that would take years. People like Coonman will posture, and the MSM will support them every step of the way, but we’ll see how long they can hold out in an effort to tank the economy if other states successfully follow the Opening Up America Plan. I seriously doubt the Blue State governors are ready for the pressure their own people would bring upon them not to play politics with their economic survival. And if that doesn’t do it, “wait until unionized Dem city workers lose their jobs, well, ” To paraphrase Donald Trump, Liberate America!!!
[Bookworm here: I just want to add to this excellent post my strong sense that Mike Pence is the driving force behind much of what Wolf Howling describes above. I can’t imagine Trump having chosen a better Vice President, or having made a better choice for someone to chair the federal task force.]